As many as 76% of cosmetic technologists believe that regulatory changes in the ingredient area require them to work a lot or very intensively, according to the Technologist Needs Survey report by CosmetoSAFE Consulting. The increasing commitment to work on updating product documentation means that specialists lack the time to create innovative products and develop new concepts. Rescue comes from CosmetoSAFE Assist, a tool for R&D departments, that will automate many processes when creating and updating product concepts.
We asked technologists about their opinions on the biggest challenges in their work today. The top three challenges in the work of technologists today are: disrupted supply chains (37% of respondents ranked the problem as the most important); keeping up with legislative changes in the area of ingredients (39% of respondents ranked the challenge as number two and 35% as number one!); and adapting products to changing market trends. Consequently, we can see that the pandemic aftermath is still being felt strongly in the R&D area, which for more than three years has been shaping
and changing the way the cosmetics industry operates in Poland and Europe.
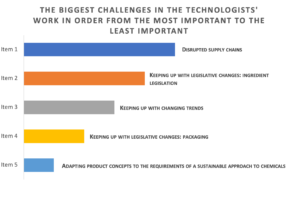
Dominance of responses on a five-point ranking scale, n = 99.
The following challenges faced by R&D departments today were mentioned among the most frequently repeated individual responses:
- seeking substitutes for raw materials on an ‘as-is’ basis,
- disrupted raw material supply chain: availability and price volatility make it impossible to forecast production costs,
- short transition periods for changes to constituent legislation; the time is too short
to develop stable formulations, - the lack of a single, consistent database and clear information on legislative changes provided well in advance.
A trend emerges from the responses of our respondents, remarks Iwona Białas, safety assessor and CEO of CosmetoSAFE Consulting, indicating that innovation, keeping up with trends, in today’s reality, has to give way to current problems related to maintaining production continuity and keeping up with legislative changes.
Having worked with cosmetic technologists for a decade years now, developing cosmetic safety assessment reports, we largely work on the same data (documents), we can see what problems they face on a daily basis. Based on this experience, we have created CosmetoSAFE Assist – this is an intuitive tool designed to facilitate the daily work of cosmetic companies’ R&D teams, Iwona Białas concludes.
What is CosmetoSAFE Assist?
CosmetoSAFE Assist is CosmetoSAFE Consulting’s proprietary tool supporting the work of technologists, product documentation specialists, regulatory departments or product managers, which comprehensively addresses product assumptions. Its aim is to increase productivity and effectiveness of work as well as minimising the risk of errors. The tool cuts by 50% the time spent working on cosmetic product documentation by:
- automation of product documentation (PIF), concentration calculations or price calculations,
- efficient management of formulations, raw material documentation and related information,
- assessing compliance with the requirements for marketing statements,
- enabling the tracking of blacklists and information on banned substances.
How do companies work with product and raw material documentation today?
The development of a cosmetic product is, on the one hand, a challenging conceptual task – we have to be inventive in terms of the claimed properties of the product, its composition, and its application properties. On the other hand, the legislator imposes on us the obligation to ensure adequate repeatability of manufacturing, stability, microbiological purity or safety of product application and, finally, evidence of the claimed cosmetic effect. The technologist’s job requires technical skills and time to perform all the necessary activities on the laboratory side. In addition, the technologist must collect, process and properly archive a lot of information: raw material dossier, test results, packaging data, etc.
The results of the survey confirm that, in the era of almost geometrically increasing legislative changes, working on product documentation takes more and more time. The matter becomes even more complicated when legislative changes require rapid implementation into product items present on the market. When asked how much time it takes them to work on product reformulations and adaptations of formulations to legislative changes, technologists indicated a decidedly greater involvement in this type of activity in relation to 2020: for 10% of respondents, product updates today take up approx. 50% of their working time (less than 4% of respondents gave such an answer in 2020); for 25% of the respondents, it is between 30 and 50% of their time (almost twice as many respondents chose this answer when compared to 2020).
Technologists note the lack of human resources to deal mainly with documentation. In particular, the constant recipe changes generate a huge amount of work related to updating the PIF or safety assessment of the finished product. An additional problem is that the documents provided by suppliers are not prepared according to a uniform template. Sometime they are incomplete or even incorrect, or are difficult to obtain. Significantly, the lack of human resources to complete the documentation is a problem indicated by 58% of respondents today, while three years ago it was 33% of respondents.
When asked about the tools and/or system solutions used for recipe creation and related duties (concentration calculations, labelling, etc.), as many as 72% of survey participants said that they only use Microsoft Excel. For mass production costing tools, 84% of respondents admitted that they use Excel for this purpose, too. Those who use other software for documentation work most often use ERP or other in-house systems.
Interestingly, there is a growing awareness among R&D employees of the possible use of automated solutions in their work. In 2020, only 6.17% of respondents complained about the lack of appropriate tools that would systemically help manage data, compared to nearly 30% today.
In our view, automation is also an opportunity for organisations to effectively manage their internal know-how. When presenting CosmetoSAFE Assist, we cannot promise that all the red-tape activities related to cosmetics creation can be transferred to data processing tools: in our solution, technologists still have to analyse the raw material dossier, properly assess the quality and suitability of raw materials or packaging to his or her requirements, but note that they perform these activities for a given item on a one-off basis (according to our proprietary data archiving system) and then use the conclusions in future implementations – until, obviously, the next change of a given item takes place, sums up Iwona Białas.




